-
- INTACT™ Sterilization
- Our closed containers technology safely and effectively enables an in-line gas sterilization method developed internally. Read more...
-
- INTACT™ Fillers
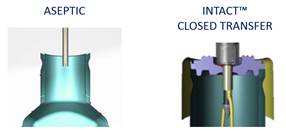
- The INTACT™ sterile Filling system, also known as the closed vial filling technology, is considered by the FDA as the paradigm shift in aseptic filling. It is today the safest filling method. Read more...
-
- INTACT™ Valve (Dispensing)
- The INTACT™ one-way valve prevents germs and air ingress, enabling non-preserved multiple-dose formulations, solutions, suspensions or creams at room temperature from first to last dose. Read more...
-
- INTACT™ Connectors
- INTACT™ Connectors: Setting a new standard in Sterile Connectors for Single-Disposable or Multiple-Use Read more...
Press Releases & News
- Satio and INTACT Form Strategic Partnership to Jointly Improve Delivery of Mass Vaccination
Business Wire – August 23, 2022
View Press Release
- Africa’s COVID vaccine project takes a step forward with bottling agreement
F. Guarascio – Reuters August 2021
View Press Release
- GE Research project in Niskayuna seeks faster vaccine production for future pandemics
J. Cropley – The Daily Gazette March 2021
View Press Release
- GE Gets $41M to Accelerate Vaccine Manufacturing, Distribution
S. McGrail, Pharmanews Intelligence – March 2021
View Press Release
- Encube Press Release
Medical Instill and Encube Ethicals, a US FDA approved and one of the largest contract manufacturers of semi-solid preparations, announce a global alliance for Intact™ Technology, end to end solutions.
Visit Encube Ethicals Website
- A first Vaccine product approved in the Closed Vial for all European countries
Synflorix™ from GSK Biologicals approved by EMA in the closed vial
View Press Release
- Viaggi wins in the Equipment Category at the Sirha 2011 Innovation Awards
View Press Release
- Viaggi Press Release
Nestlé Viaggi, launched in Europe in June 2010 by Nestlé, became thanks to Medical Instill’s Intact™ one-way non contamination Valve the first fully integrated, automatic espresso and cappuccino machine with ambient storage of milk and chocolate concentrate.
View Press Release
- LACF No Objection for Intact™ Valve
The Intact™ dispensing valve received no objection at the Low Acid Canned Food (LACF) office of the US FDA (SID: 2009-04-02/001 and 2009-04-06/001)
- Aseptic Technologies wins 2009 Facility of the Year Award Winner for Equipment Innovation
View Press Release
- Nestle Press Release
Nestlé Nutrition and Medical Instill announce Exclusive Partnership Agreement to manufacture sterile liquid nutrition products.
View Press Release
- GSK Press Release
Medical Instill and GlaxoSmithKline Biologicals announce long term deal on Intact™ for single-dose vaccines and injectables.
View Press Release
- In June 2011, the first vaccine using the closed-vial technology for primary packaging was approved by the EU.
-

James AGALLOCO, "Closed Systems and Environmental Control: Application of Risk Based Design"
American Pharmaceutical Review, July 2004
View PDF -
E. Greb, "In Search of a Sterile Fill"
Pharmaceutical Technology 34 (3) 2010
View PDF -

D. Py and A. Turner, "Genesis of the closed vial technology"
Advanced Aseptic Processing Technology (editors J. P. Agalloco and J. Akers)
Informa Healthcare publication 2010
View PDF -

D. Hussong et. al, "Closed system Filling Technology: A New Paradigm"
PDA Letter November/December 2015
View PDF -

M. Lal et. al, "Stability of live attenuated rotavirus vaccine with selected preservatives and primary containers"
Vaccine. 34 (22) 2016
View PDF -
J. Agalloco and L. Mestrandrea, "Moving to Closed Systems for Aseptic Processing"
Pharmaceutical Technology and BioPharm International Vaccine Development and Manufacturing 2017 eBook (November 2017)
View PDF -

T. Scherder et. al, "An Evaluation of a Closed Sterile Transfer Process for Aseptic Filling"
Pharmaceutical Online July 21 2017
View Article -

F.A. Toba et. al, "Establishing Correlation between Aerosol and Surface Microbial Populations"
Pharmaceutical Technology 42 (2) 2018
View PDF